If you have suffered an injury or a loved one has died as the result of over or under infusion of fluids while an Alaris Medley infusion pump was being used, you may have a case against Elite Biomedical Solutions and Alaris Medical Systems. If you are considering filing a lawsuit, contact us today for a free no obligation case evaluation.
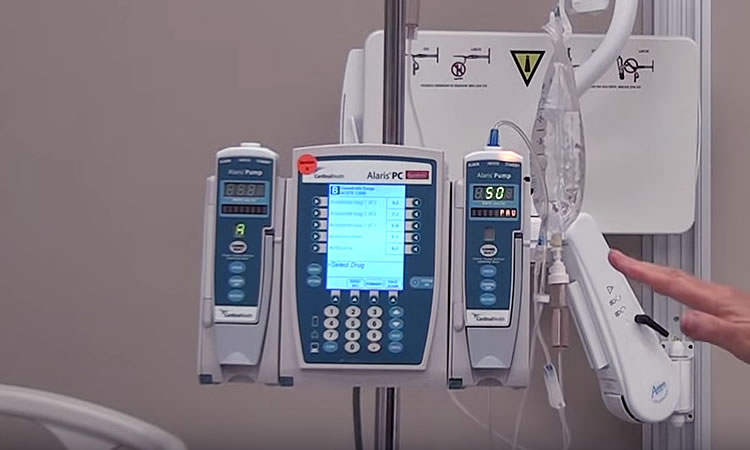
Infusion pumps are machines designed to continuously or intermittently administer fluids, medication, or blood and blood products to patients in ways that might be impractical or too expensive if performed by nursing staff. For example, infusion pumps can automatically administer very tiny amounts at regular intervals, or can continuously deliver fluids such as nutrients, into the patient’s body in controlled amounts. Because they also have the capability of injecting controlled amounts of fluid beneath the skin (subcutaneously) as well as within the surface of the central nervous system (epidurally), they have become popular for use in administering local spinal anesthesia in childbirth. These pumps are now in widespread use both in medical settings and in homes.
While these pumps have made the infusion process easier and more convenient, and have been a major factor in the growth of home infusion in particular, they have long been known to be a source of adverse patient events. For this reason, the FDA monitors reports of such events and keeps track of problems with medical devices like these in order to alert both health professionals as well as the public when it comes to safety concerns of patients.
FDA Recalls Alaris Medley Large Volume Pump
Device: Alaris Medley Large Volume Pump (LVP) Frame Membrane
- Part and Lot numbers:
- Frame Membrane NOEM, Part # TC10006587 / 10013801 (Lot # 022015502 and 0421151000)
- Frame Membrane NOEM Shipped with LVP Bezel Assembly, Part # 49000204 (Lot # 031815100, 032415100 and 043015215)
- Manufactured from: February 20, 2015 to April 21, 2015
- Distributed from: February 25, 2015 to May 8, 2015
- Devices Recalled in the U.S.: 1,502
In June of 2015, the FDA ordered a Class I recall of one such device. A Class I recall is the most serious type of recall as there is reasonable probability that the use of or exposure to a product will result in a very serious adverse health outcome, including death. In other words, Class I recalls carry the most serious consequences (serious injury and even death).
On May 21, 2015 Elite Biomedical Solutions sent Product Advisory Notices to their customers, and then on June 3rd, issued them Urgent Medical Device Recall letters, stating they had found that the Alaris Medley Large Volume Pump (LVP) had a part that could result in an over or under infusion of fluids to patients that could result in injury or death. The frame membranes that are part of the pump and are to prevent fluids from leaking into the internal components was found to be defective. On June 12, 2015 a press release was issued to all hospitals in the U.S. via the Emergency Care Research Institute (ECRI) concerning this product. The notification letter asked customers to:
- Inspect and quarantine all affected products
- Identify customers and notify them immediately of the product recall
Hospitals were also asked to complete and return a form that had been enclosed with the notice and to contact Elite Biomedical Solutions. Finally, both healthcare professionals and patients were encouraged to report any adverse events or side effects resulting from use of this product.
Infusion Pump Safety Concerns Over the Years
Infusion pump safety concerns are not a new problem. Between 2005 and 2009 alone there were over 56,000 adverse events due to such infusion pump issues. During that same time period, 87 infusion pumps recalls were conducted by firms to address identified safety problems. At that time, 70 of the recalls were designated Class II, which means the device may be the cause of a temporary or medically reversible adverse health outcome, but 14 were Class I. It was for that reason the FDA initiated the Infusion Pump Improvement Initiative in 2010.
Get Help from an Infusion Pump Lawyer
While the initiative proposed stricter regulations of these devices, it cited such issues as software defects, user interface problems, and mechanical or electrical failure as the chief causes of serious or potentially adverse events. Clearly, these problems still exist and manufacturers of these devices need to be held accountable for compromising patient safety. If you or a loved one was harmed as a result of the Alaris infusion pump delivering an over or under infusion of fluids, contact us today so we can review your information and connect you with the right attorney. There is no fee for this and if your case is accepted, you pay no attorneys fees at all unless they win your case.
References
FDA. (2015). Elite biomedical solutions, Alaris medley large volume pump (LVP) frame membrane: Frame membrane may allow over or under delivery of fluid by an infusion pump. http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm460079.htm
FDA. (2010). Infusion pump improvement initiative. Center for devices and radiological health. http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/%5CparGeneralHospitalDevicesandSupplies/InfusionPumps/UCM206189.pdf
Infusion Pumps. https://en.wikipedia.org/wiki/Infusion_pump#cite_note-2
Other Resources
FDA recent medical device recalls and other FDA safety communications. http://www.fda.gov/MedicalDevices/Safety/



No Comment