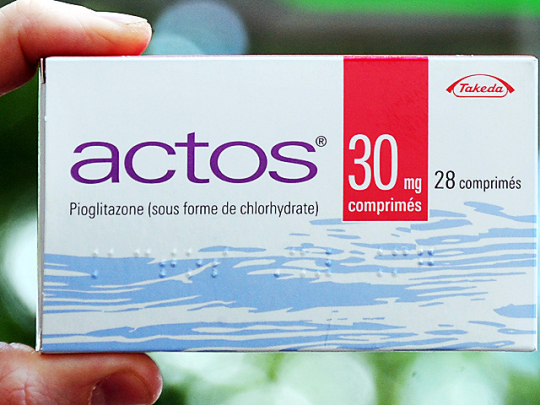
Were you prescribed Actos as a treatment for your type 2 diabetes and are now suffering from bladder cancer or other problems? You may have a lawsuit against the makers of Actos. The FDA has announced there has recently been a 40% increase of bladder cancer that is linked the the prescription drug Actos.
Actos is designed for type 2 diabetes patients and works by helping the cells become more sensitive to a hormone that is produced by the pancreas, called insulin. Insulin helps throughout the body to regulate glucose(sugar) levels. In hope of making the cells more sensitive to the insulin, Actos is supposed to allow the glucose to move easily throughout the cells, to have proper regulation of glucose & sugar levels throughout the body.
Amongst bladder cancer, Actos has also been linked to other serious health complications including lactic acidosis, bone fractures, macular edema, congestive heart failure, and many other side effects. If you or a loved one have suffered any of these injuries or complications after taking Actos, you may be able to receive compensation to help with the pain, suffering and to cover your current and any other future medical bills.
If you have experienced any of these symptoms contact a doctor immediately:
- Blood in urine
- Painful urination
- The increased urge to urinate
- Back pain that is unusual
Black Box Warning Information from the FDA
Bladder Cancer
The FDA updated their concerns about Actos and the increased risk of bladder cancer association in August 2011. Patients with a history of bladder cancer, or currently have bladder cancer should not use Actos.

No Comment